The Radical Redox-Relay Strategy
Radical-based chemistry has long been a cornerstone of organic synthesis. However, catalyst-controlled stereoselective reactions involving free radical intermediates remain limited, and the discovery and control of such processes is highly desirable in the construction of complex structural motifs. Our research efforts in radical catalysis have focused on the design of novel chiral catalysts that can generate and stabilize radical intermediates while enforcing stereo- and regiocontrol needed for complex transformations. Important features of our redox-relay strategy include precise control over the (1) formation of radical species, (2) the transformation of these species into new radical intermediates, and (3) the termination of radical events to form the desired product. Our strategy has given rise to stereoselective methods for the preparation of valuable synthetic intermediates such as polysubstituted pyrrolidines, cyclopentanes, and allylic alcohols.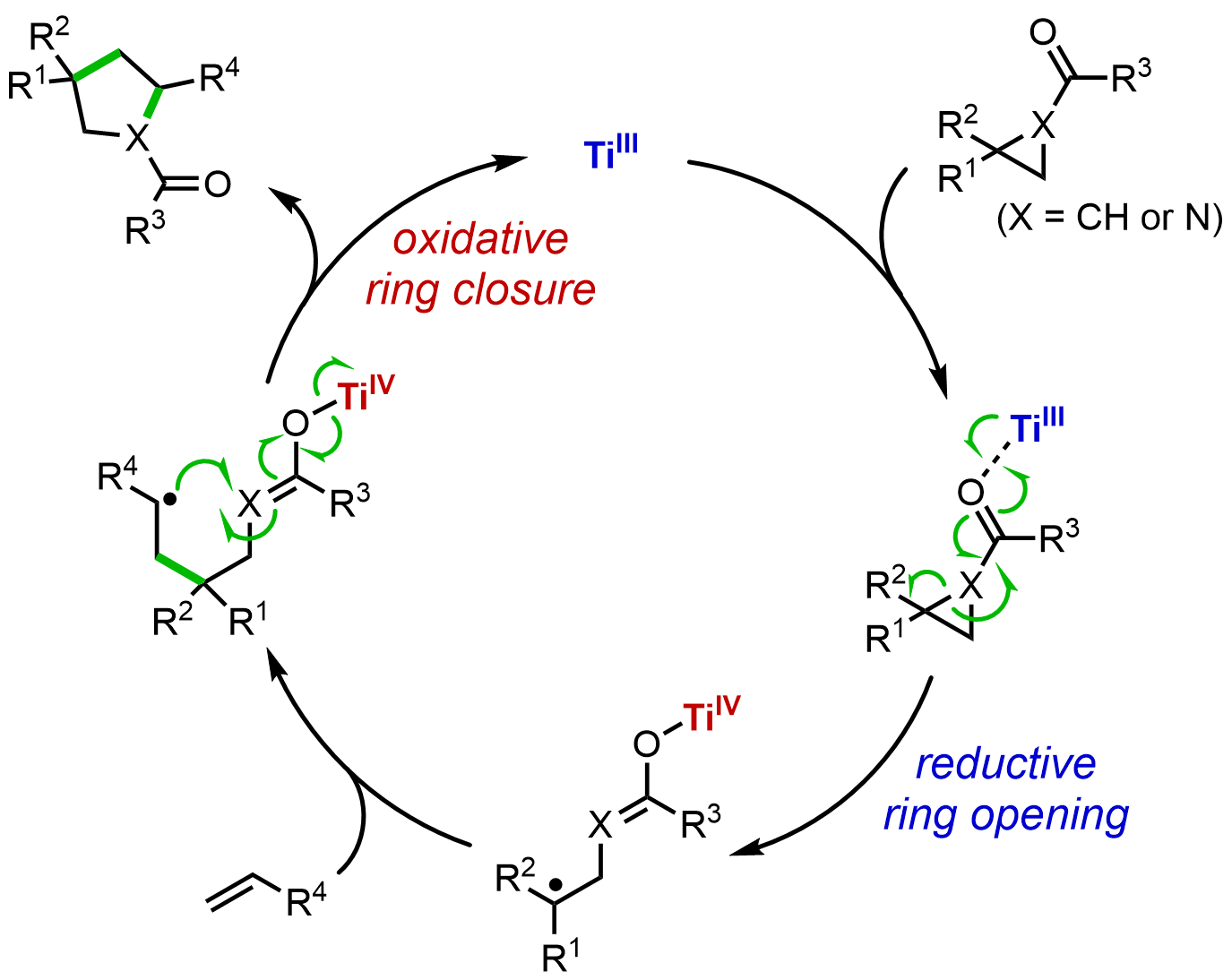
Representative Publications
Wu, X.; Chang, Y.; Lin, S.* “Titanium Radical Redox Catalysis Reactions, and Modes of Activation.” Chem 2022, 8, 1805–1821. DOI: 10.1016/j.chempr.2022.06.005 [html] Invited perspective. Dedicated to the occasion of Prof. John McMurry’s 80th birthday

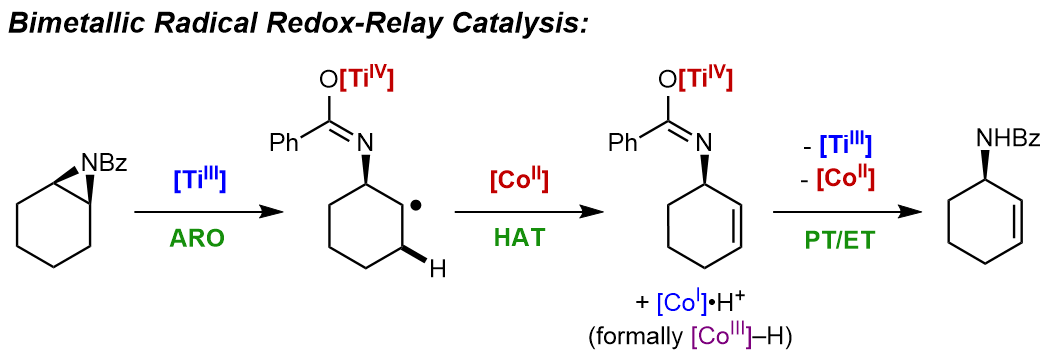
Wood, D. P.; Guan, W.; Lin, S.* “Titanium and Cobalt Bimetallic Radical Redox Relay for the Isomerization of N-Bz Aziridines to Allylic Amides.” Synthesis 2021, 53, 4213–4220. DOI: 10.1055/s-0037-1610779 [html]
Robinson, S. G.; Wu, X.; Jiang, B.; Sigman, M. S.; Lin, S. “Mechanistic Studies Inform Design of Improved Ti(salen) Catalysts for Enantioselective [3+2] Cycloaddition” JACS 2020, 142, 43, 18471–18482. DOI: 10.1021/jacs.0c07128 [html]
McCallum, T.; Wu, X.; Lin, S. “Recent Advances in Titanium Radical Redox Catalysis” JOC 2019, 84, 14369-14380. DOI: 10.1021/acs.joc.9b02465 [html]
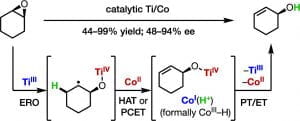
Ye, K-Y.; McCallum, T.; Lin, S. “Bimetallic Radical Redox-Relay Catalysis for the Isomerization of Epoxides to Allylic Alcohols” J. Am. Chem. Soc. 2019, 141, 9548-9554. DOI: 10.1021/jacs.9b04993 [html]
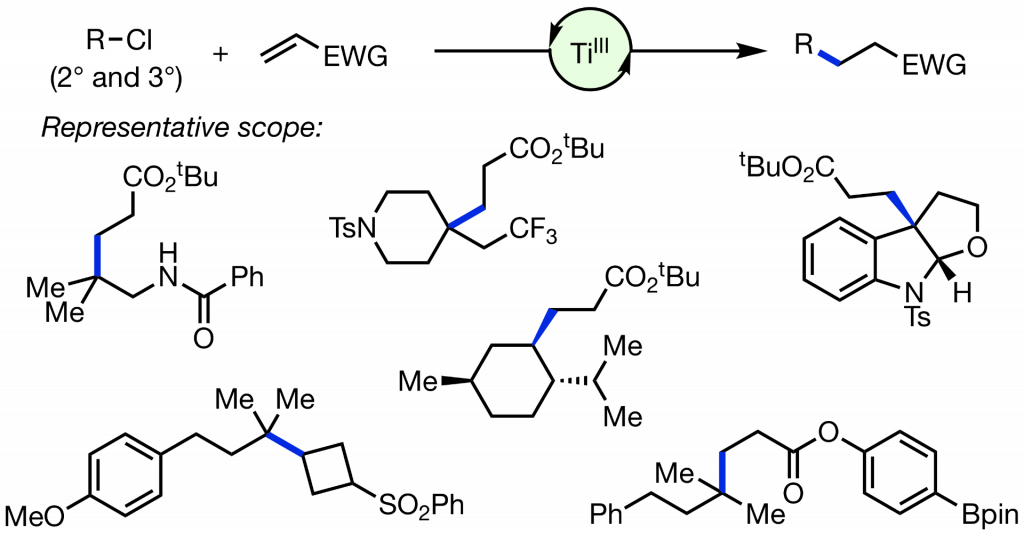
Wu, X.; Hao, W.; Ye, K-Y.; Jiang, B.; Pombar, G.; Song, Z.; Lin, S. “Ti-Catalyzed Radical Alkylation of Secondary and Tertiary Alkyl Chlorides Using Michael Acceptors” J. Am. Chem. Soc. 2018, 140, 14836-14843. DOI: 10.1021/jacs.8b08605 [html]
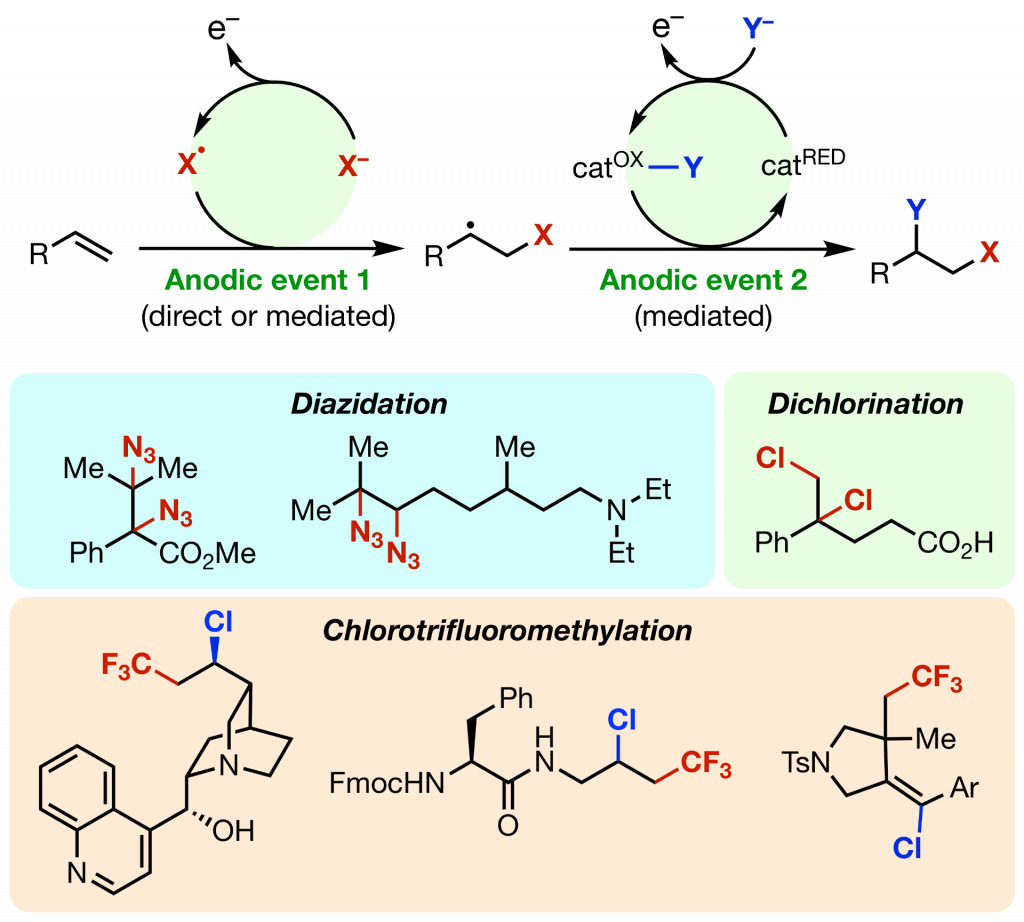
Sauer, G. S.; Lin, S. “An Electrocatalytic Approach to the Radical Difunctionalization of Alkenes.” ACS Catal. 2018, 8, 5175-5187. DOI: 10.1021/acscatal.8b01069 (Invited Perspective). [html]
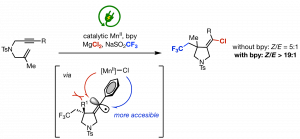
Ye, K-Y.; Song, Z.; Sauer, G. S.; Harenberg, J. H.; Fu, N.; Lin, S. “Synthesis of Chlorotrifluoromethylated Pyrrolidines by Electrocatalytic Radical Ene‐Yne Cyclization.” Chem. Eur. J. 2018, 24, 12274-12279. DOI:10.1002/chem.201802167 (Young Chemists Special Issue). [html]
Hao, W.; Harenberg, J. H.; Wu, X.; Macmillan, S. N.; Lin, S. “Diastereo- and Enantioselective Formal [3 + 2] Cycloaddition of Cyclopropyl Ketones and Alkenes via Ti-Catalyzed Radical Redox Relay” J. Am. Chem. Soc. 2018, 140, 3514–3517. DOI: 10.1021/jacs.7b13710 [html]

Hao, W.; Wu, X.; Sun, J. Z.; Siu, J. C.; MacMillan, S. N.; Lin, S. “Radical Redox-Relay Catalysis: Formal [3+2] Cycloaddition of N-Acylaziridines and Alkenes”, J. Am. Chem. Soc. 2017, 139, 12141-12144. DOI: 10.1021/jacs.7b06723. [html]
Highlighted in SynForm


