Electrophotocatalysis in the Lin Lab
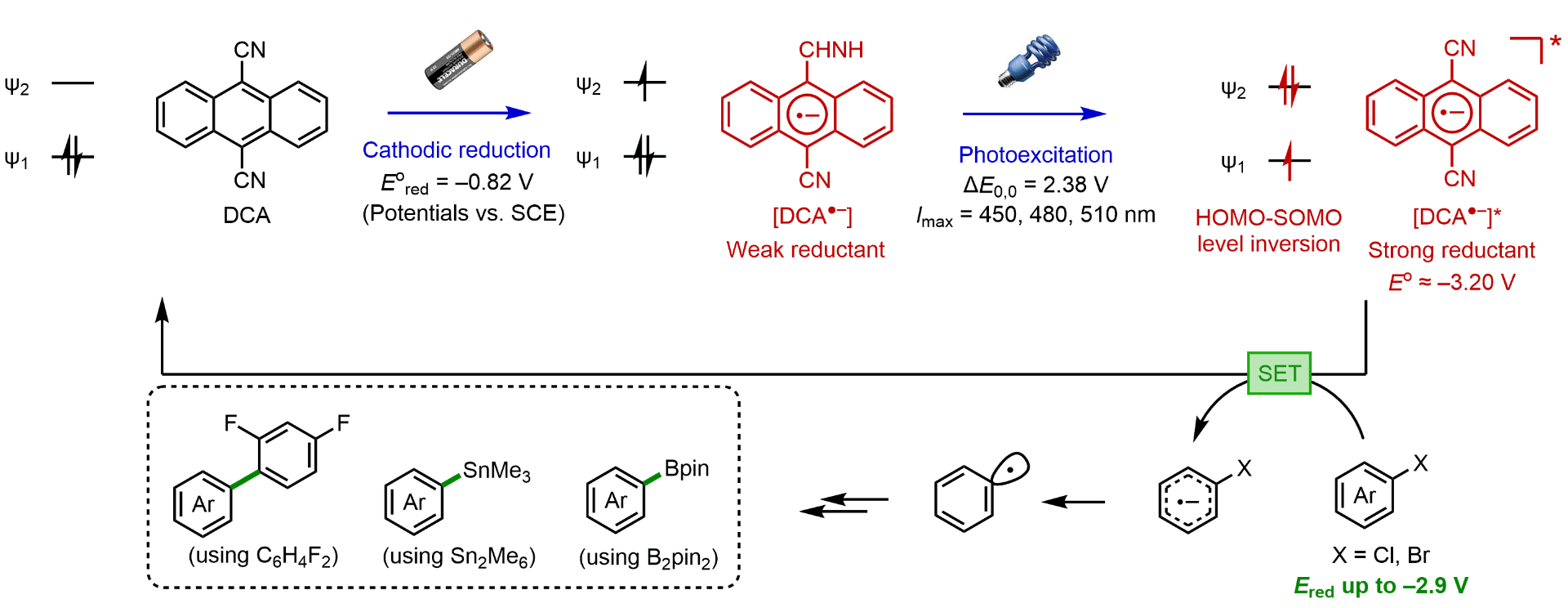
The development of catalytic transformations driven by single-electron processes is an area of intense research in organic chemistry. In particular, the use of unconventional means of activation, including photoredox catalysis and electrocatalysis, has provided unique access to such reactivities and has produced new solutions to challenging synthetic problems. Photoredox chemistry and electrochemistry display complementary characteristics in synthetic contexts. Photoredox catalysis offers access to unique chemical reactivities by using the excited states of organic molecules for overall redox-neutral transformations. By using electricity as a traceless redox agent, electrochemical methods can be used to render photoredox systems capable of net oxidation or net reduction. We have developed several strategies that harness the potential of this synergistic system to access catalytic intermediates with high redox potential in a transient, controlled fashion in the absence of a chemical oxidant or reductant.
Representative Publications
Liu, J.; Lu, L.; Wood, D.; Lin, S. “New Redox Strategies in Organic Synthesis by Means of Electrochemistry and Photochemistry” ACS Cent. Sci. 2020. DOI: 10.1021/acscentsci.0c00549 [html]

Zhang, W.; Carpenter, K.; Lin, S. “Electrochemistry Broadens the Scope of Flavin Photocatalysis: Photoelectrocatalytic Oxidation of Unactivated Alcohols” Angew. Chem., Int. Ed. 2020, 59, 409-417. DOI: 10.1002/ange.201910300 [html]

Kim, H.; Kim, H.; Lambert, T.; Lin, S. “Reductive Electrophotocatalysis: Merging Electricity and Light to Achieve Extreme Reduction Potentials” JACS 2020, 142, 2087-2092. DOI: 10.1021/jacs.9b10678 [html] For the ChemRxiv preprint version, see [html]